Hits: 150
Race for the next generation of Covid-19 vaccines
What’s in the pipeline?
WPCNR EDITOR’S LOCAL NOTES: LOCALLY, COVID INFECTIONS ARE A LURKING FACTOR IN WESTCHESTER. HOSPITALIZATIONS IN THE MID HUDSON REGION WERE 63 COMPARED TO 81 LAST YEAR ON APRIL. WESTCHESTER COUNTY HAD THE MAJORITY OF THOSE CASES .
FROM APRIL 9 TO APRIL 17, WHITE PLAINS HOSPITAL SAW 50 PERSONS ADMITTED AND 15 OF THEM POSITIVE FOR COVID, 30%. THAT WAS UP A LITTLE FROM 25% OF ADMISSIONS FOUND WITH COVID LAST WEEK WHEN 9 OF 36 ADMISSIONS TESTED POSITIVE FOR COVID.
THE COUNTY AVERAGE 2.8 INFECTIONS A DAY FROM APRIL 7 TO 13, 196 IN A WEEK WHICH LAST WEEK ACTUALLY REACHED 216.
IF WE CONTINUE THAT PACE APRIL WOULD HAVE 868 FOR THE MONTH. IN 2023, APRIL SAW 895 COVID CASES SO WESTCHESTER HAD ONLY 4% OR 27 INFECTIONS DOWN LAST APRIL WHEN INFECTIONS STARTED TO REBOUND UPWARDS INTO JUNE.
THE PASSOVER OBSERVANCE HAPPENING LAST YEAR IN WESTCHESTER COMBINED WITH THE RELIEF OF COVID RESTRICTIONS PRODUCED 814 COVID CASES IN MAY, 796 IN JUNE, RESULTING IN AN EXPLOSION OF CASES IN JULY 2023 1,064 AND 1,962 IN AUGUST. WHAT WILL MAY AND JUNE SOCIALIZATION DO?
THE NUMBER OF CASES NOW IS CLIMBING BACK TO 1,000 CASES A WEEK.
WILL COVID COME BACK?
AT 860 CASES NOW IT MIGHT COME BACK FOR A 7TH WAVE AND LAUNCH ANOTHER MISERABLE COVID SURGE.
PLEASE SOCIALIZE RESPONSIBLY. FOR THE RECORD LAST WEEK WAS THE 10TH IN 11 WEEKS IN THE LAST 2-1/2 MONTHS WE LOWERED THE CASES ( BUT ONLY 1 LESS THAN LAST WEEK. NOW TO DR. KATELYN JETELINA’S LATEST EDITION OF YOUR LOCAL EPIDEMIOLOGIST:
We could definitely use better Covid-19 vaccines. While our current ones work well against severe disease, they could be better in other departments, like:
- Stopping transmission
- Covering future mutations
- Having fewer side effects
- Ability to combine with other vaccines, like the flu vaccine
A suite of vaccines—considered next-generation (NextGen)—are in the clinical trials pipeline. While some are showing positive results, there is still a long road ahead.
Where are they in the process? Let’s dig in.
Note: We tried our darndest to summarize this vaccine pipeline comprehensively, but this stuff is hard to find. This list could be incomplete or (a little) out of date.
A quick reminder
Vaccines undergo a long process to ensure they are safe and effective, from preclinical (i.e., testing in animals) to phase III (testing in hundreds of people) before authorization by the FDA. About 9 of 10 products fail at some point in this process. A typical vaccine takes 5 to 10 years—that is, when we aren’t in a massive emergency (with lots of money and the entire world working towards one thing).
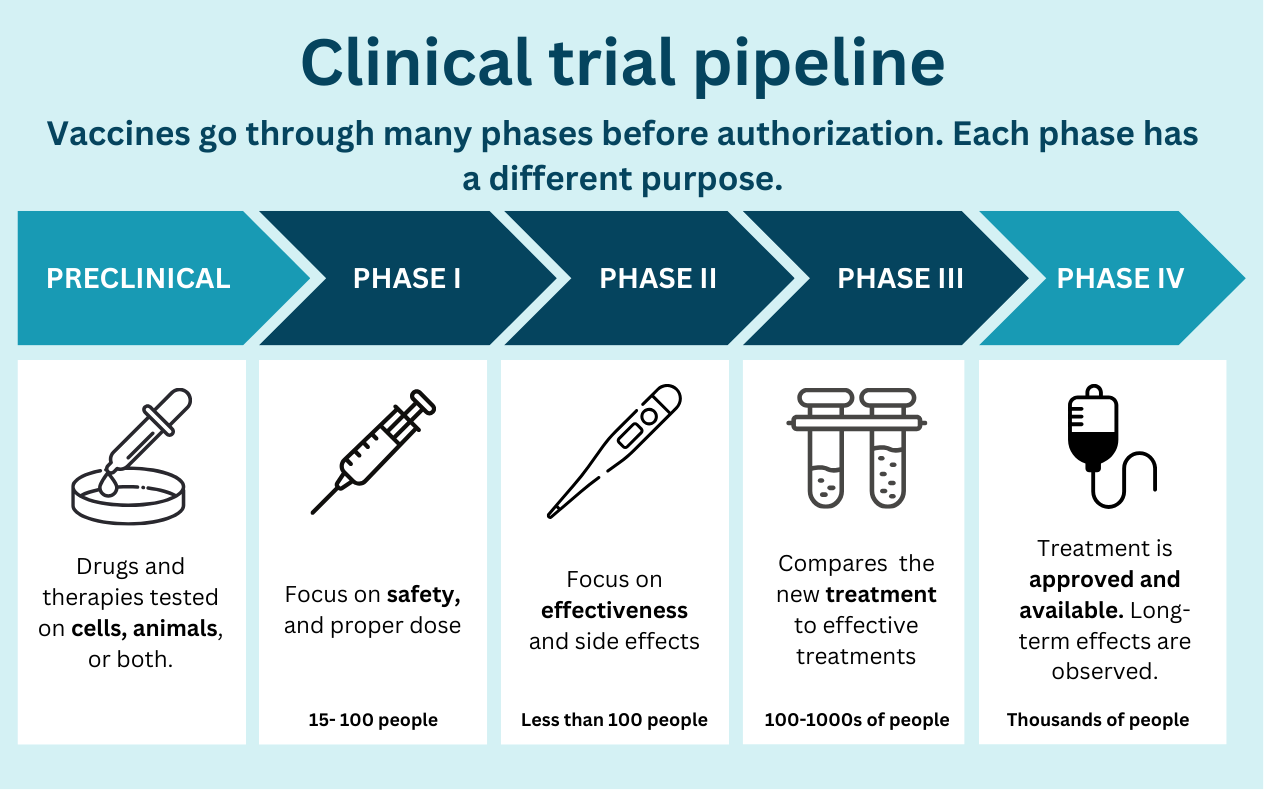
While the U.S. government gave some NextGen vaccines a big financial boost at the end of the emergency ($5 billion), it will likely still be a while before we get them in arms. These vaccines are truly novel, and we are still trying to figure out how they work best.
NextGen Category #1: Variant-proof vaccines
One NextGen solution is a universal coronavirus vaccine that would protect against not only SARS-CoV-2 but also other coronaviruses that might cause future animal outbreaks and pandemics. However, this is a long way away.
We have made progress towards a pan-Covid-19 vaccine. This class of vaccines aims to be “variant-proof.” The idea is that these vaccines would induce an immune response that would make it impossible (or at least very difficult) for newer variants to escape antibodies, like Omicron did in 2021. This doesn’t necessarily mean that we would no longer need boosters or that these vaccines could stop transmission. Only time would tell us that.
Around 20 variant-proof vaccines are in the early stages of this process (preclinical), but 5 have reached human trials.
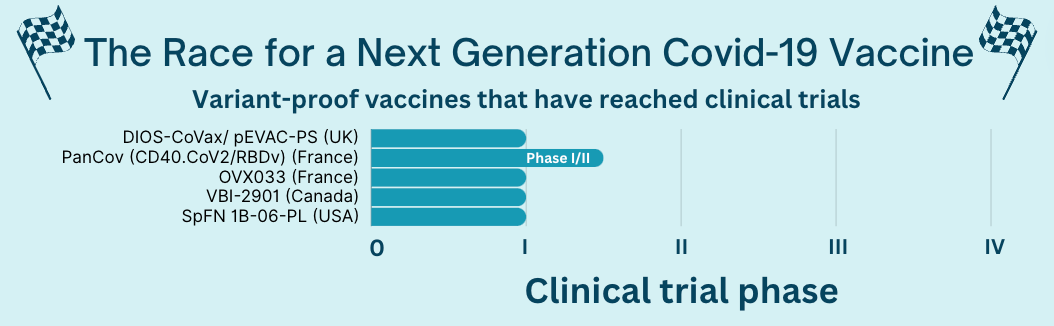
- For example, the VBI vaccine (made by a Canadian company partnered with the Walter Reed Army Institute of Research) induced an immune response to many Covid-19 variants and coronaviruses, but the data is limited. Walter Reed described its vaccine as having “positive” effects, but no detailed data have been released yet.
NextGen Category #2: Mucosal Vaccines
The next category is mucosal (i.e., nasal) vaccines. These induce antibodies in a person’s nose and throat—the major site of infection by SARS-CoV-2—so they attack the virus at the starting line. Theoretically, this would better prevent infection and transmission than current vaccines. As previously covered on YLE, this is a hard scientific road for multiple reasons.
Twenty-seven clinical trials of mucosal vaccines have reached human trials, including a few in the U.S. A lot are still in the beginning stages, though.
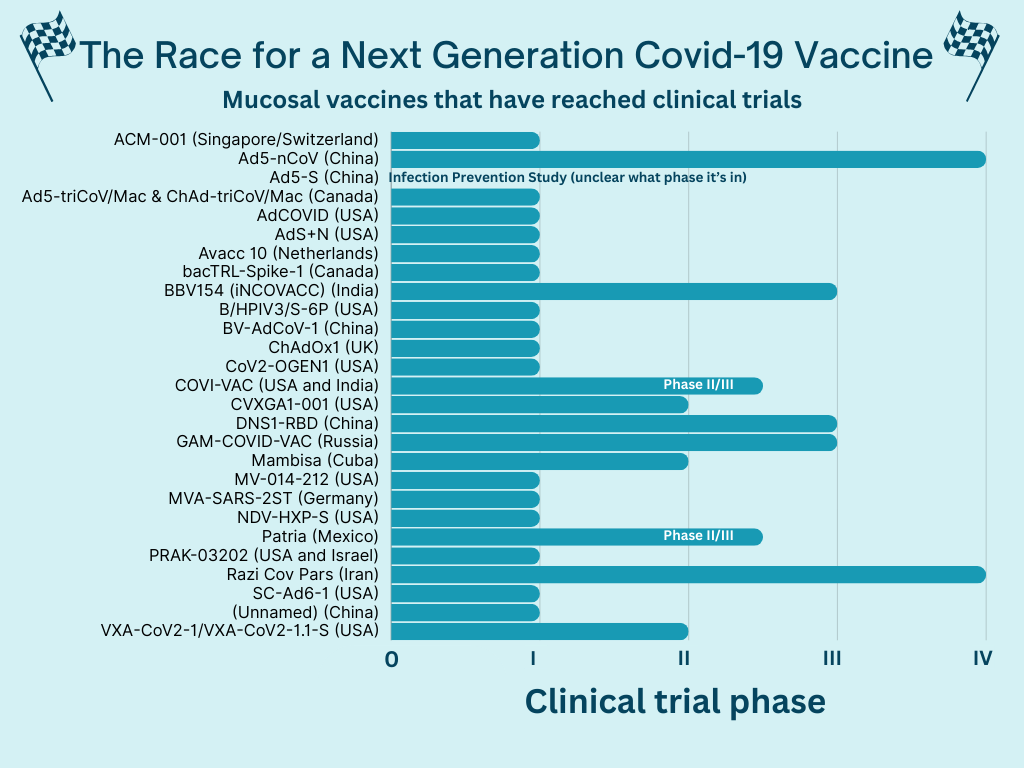
A few have reached later phases, and some have even been approved in other countries. However, they haven’t been authorized by a drug regulatory agency considered “stringent” for the WHO or the U.S. In the U.S., these manufacturers would have to submit their materials to the FDA and, after review, may have to run another clinical trial if they don’t have certain data. It’s not clear if this is happening (or not).
Some promising late-stage results so far include:
- CanSino, a Chinese vaccine company, published their intranasal vaccine results as “highly effective.” In another phase IV trial, the intranasal vaccine had a slower immune response than the injected vaccine but lasted longer.
- Bharat Biotech’s (India) intranasal vaccine elicited a higher immune response than the injected vaccine in phase III clinical trials.
NextGen Category #3: Everything else
Combining Covid-19 vaccine with the flu vaccine. Moderna, Novavax, and Pfizer are all working on a flu+Covid vaccine. Some are further along than others. Some manufacturers hope this will be available in the 2024 fall season. I’m skeptical.
Self-amplifying mRNA. This vaccine would include instructions on how to replicate RNA, once inside our body, allowing for much lower doses of injected mRNA. This means, in theory, fewer unpleasant side effects. There are two in the pipeline:
- In Japan, a self-amplifying mRNA (samRNA) showed better immune responses against Omicron BA.5 than the Pfizer vaccine as a booster and has been approved for use there. This was endorsed by WHO.
- Gritstone Bio (U.S.) is also working on one. They plan to study their vaccine in a phase 2b trial later this year.
Don’t forget about T cells! Some have developed vaccines to maximize our T cell responses by targeting other parts of the coronavirus (not the spike). These vaccines wouldn’t have much effect on transmission or infection, but they could be very valuable in preventing hospitalizations if/when we get another coronavirus pandemic.
- Pfizer and BioNTech have reported preclinical data, which showed robust immune responses to non-spike targets.
- The big challenge with this approach is manufacturers would need an absolutely huge trial (given the outcome is hospitalizations). They could try to justify the vaccine based on the T cell responses, but we don’t know how to interpret those in terms of real-world outcomes. This will take some work.
Bottom line
We need better Covid-19 vaccines. Biomedical innovation, such as the licensure of mRNA vaccines, was a huge scientific win during the emergency, and thankfully, scientists are not stopping there. While these will not likely be available this year, we can cross our fingers and toes that better ones are on the horizon.
Love, YLE and Andrea
Andrea Tamayo is an intern at YLE. She is a science journalist and master’s student at the University of California, Santa Cruz Science Communication Program. You can find more of her stories at andreactamayo.com.
Big thanks to Edward Nirenberg, who verified some of the data and scientific translations. And he didn’t let us forget about the cool work with T cells.
“Your Local Epidemiologist (YLE)” is written and founded by Dr. Katelyn Jetelina, M.P.H. Ph.D.—an epidemiologist, wife. During the day, she is a senior scientific consultant to several organizations, including CDC. At night, she writes this newsletter. Her main goal is to “translate” the ever-evolving public health world so that people will be well-equipped to make evidence-based decisions. This newsletter is free, thanks to the generous support of fellow YLE community members. To support this effort, subscribe below:


